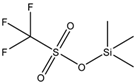
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) is an organosilicon compound with the formula (CH3)3SiO3SCF3. It is a colorless moisture-sensitive liquid. It is the trifluoromethanesulfonate derivative of trimethylsilyl. It is mainly used to activate ketones and aldehydes in organic synthesis.
Reactions
TMSOTf is quite sensitive toward hydrolysis:
- (CH3)3SiO3SCF3 H2O → (CH3)3SiOH HO3SCF3
It is far more electrophilic than trimethylsilyl chloride.
Related to its tendency to hydrolyze, TMSOTf is effective for silylation of alcohols:
- (CH3)3SiO3SCF3 ROH Et3N → ROSi(CH3)3Si [Et3NH]O3SCF3
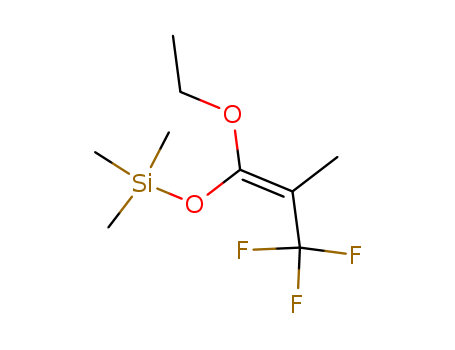
A common use of (CH3)3SiO3SCF3 is for the preparation of silyl enol ethers. One example involves the synthesis of the silyl enol ether of camphor:
It was also used in Takahashi Taxol total synthesis and in chemical glycosylation reactions.
Trimethylsilyl trifluoromethanesulfonate has a variety of other specialized uses. It has been used to install tert-alkyl groups on phosphine (R = alkyl):
- PH3 R3C–OAc Me3SiOTf → [(R3C)2PH2]OTf
Deprotection of Boc-protected amines can be achieved using trimethylsilyl trifluoromethanesulfonate and triethylamine or 2,6-lutidine.
TMSOTf is also a useful reagent to replace metal-halogen bonds with a covalent M-O(SO2CF3) bond, the by-product being the highly volatile TMSCl which is easily removed.
References